FIMICS PANEL
a long non-coding RNA panel to better characterize cardiac disorders and toxicities
FIMICS is the result of years of research on cardiac lncRNAs in cooperation with several research institutes of excellence and the collaboration with Celemics, a Seoul based biotech company specialized in Next-Generation Sequencing solutions. LncRNAs are a highly tissue specific family of RNA opening new horizons for diagnostic and therapeutic applications in the cardiovascular field. Identification of cardiac enriched lncRNAs were performed using deep sequencing human cardiac biopsies of failing and control hearts. Expression levels were compared to 12 others organs to select cardiac enriched lncRNAs.
A total of 3233 lncRNAs including 1036 novel lncRNAs never previously described were identified and included into FiMICS panel. Detection and precise quantification of these cardiac enriched lncRNAs in peripheral blood using FiMICS provide first-class results to the final users and opens new ways for therapeutics and precision medicine tools for diverse primary and secondary cardiac diseases, cardiac toxicities and drug development.
Features & Benefits
-
First in class test for the measurement of cardiac enriched lncRNA in peripheral blood
-
Proprietary list of cardiac enriched lncRNAs coming from years of research
-
More than 55.000 capture probes for high coverage of lncRNAs sequences
-
Investigation of a novel class of biomarkers/therapeutic targets
-
Get direct insight of heart condition using blood without needing cardiac biopsies.
-
Characterization of patients with diverse cardiac disorders and toxicities
High detection rate of cardiac enriched lncRNAs in peripheral blood and first-class performaces
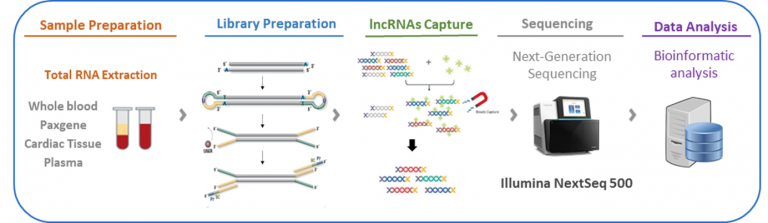
FiMICS lncRNAs analysis workflow: sample to results
-
Non-invasive samples to investigate cardiac enriched lncRNAs
-
PAXgene and cardiac tissue already validated.
-
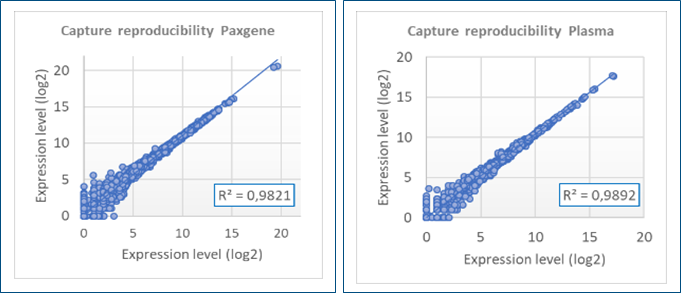
Excellent analytical performance with high reproducibility of the results
-
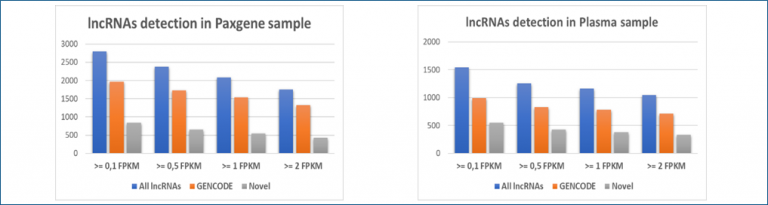
High sensitivity with ~2800 lncRNAs detected in PAXgene samples over 3233 (FPKM>0,1)
-
Plasma samples in validation process with more than 1500 lncRNAs detected (FPKM>0,1)
available here : FiMICS Datasheet
We support our clients to optimize each step of their biomarker research and development programs, from concept to regulatory qualification for all international markets.
Our team has significant experience in the regulatory qualification of biomarker-based panels and in vitro diagnostics (IVDD) tools in Europe (development and submission for CE marked kits) and in the US (IVDD and LTD kits) according to EMA and FDA guidelines.
Use our expertise in analytics, regulatory strategy, and in biospecimens solutions for comprehensive support across your research needs:
-
Design of biomarker program and analytical development
-
Planning and study design
-
Development of Standard Operating Procedures (SOPs)
-
Regulatory support: preparing dossiers and opinion meeting
-
Biomarker regulatory qualification for IVDD development
Contact us at sales@firalis.com to get further details and to discuss your projects.