MMP9 ELISA: Matrix Metalloproteinase-9 (Draft)
INSTRUCTIONS FOR USE
For quantification of MMP-9 in serum samples
 REF FLS-BP09
REF FLS-BP09
This manual must be read entirely before using this product.
For research use only (RUO). Do not use for diagnostic procedures.
Version: 1.0
|
Firalis S.A. 35 rue du Fort 68330 – Huningue FRANCE |
Phone: +00 33 389 910 111 Email: contact@firalis.com Web: www.firalis.com |
![]()
|
Table of contents |
|
Background and Significance |
|
Materials provided & Storage conditions |
|
Other material required |
|
Sample collection, Storage and Preparation |
|
Reagent preparation |
|
Assay procedure |
|
Calculation & Interpretation of Results |
|
Technical Advice |
|
Warnings and Methods Limitations |
|
Precaution |
|
Performance Characteristics |
|
Symbol Legend |
|
References |
Intended Use
Firalis MMP-9 ELISA kit is intended to be used for the in vitro quantification of human MMP-9 in serum samples.
- The assay is intended for research use only.
- The total assay time is less than 5 hours.
- The assay measures MMP-9 in serum samples.
- The format is 96 wells.
- Components of the kit are provided ready to use, lyophilized, concentrated or to reconstitute (see table of MATERIALS PROVIDED).
Background and Significance
Matrix Metalloproteinase-9 (MMP-9) is part of the matrix metalloproteinase family, a group of structurally related zinc-containing proteolytic enzymes. These enzymes play an important role in tissue remodeling of extracellular matrix. MMP-9 is a 92 kDa protein with a sequence length of 707 amino acids that is produced by epithelial cells and is stored in the secretory granules of neutrophils and eosinophils. MMP-9 is involved in vascular leakage, migration of inflammatory cells and wound repair. Several pre-clinical studies suggest that MMP-9 has the potential to predict, diagnose and prognosticate cardiac remodeling and related events. In fact, it has been shown that MMP-9 null mice that suffered from myocardial infarction (MI), were protected against cardiac rupture and demonstrated a decrease in collagen and macrophage infiltration in cardiac tissue that resulted eventually in a decrease in left ventricular (LV) dimensions, a hallmark of cardiac remodeling1, 2, 3, 4, 5, 6.
Principle of Assay
The Firalis Assay MMP-9 is a sandwich ELISA (Enzyme Linked Immunosorbent Assay) plate-based assay. The 96-wells plate is already coated with the monoclonal capture antibody specific for the soluble form of “MMP-9”. Standards, Quality controls (QC) and diluted samples are incubated in the pre-coated wells. MMP-9 present in the solutions will bind to the capture antibody while unbound and/or weakly bound substances are removed in washing steps. Then, a biotinylated secondary monoclonal antibody is added to each well, followed by another washing step to remove unbound antibody. Next, the streptavidin conjugate horseradish peroxidase (HRP) is added in each well and allowed to form a complex with the bound biotinylated antibodies during the incubation time. A third washing step is performed to remove unbound HRP-streptavidin and the enzyme substrate TMB (Tetramethylbenzidine) is added into the wells. The TMB is oxidized by HRP in the presence of hydrogen peroxide, turning TMB blue. The intensity of the colour produced is proportional to the MMP-9 amount present in each well. Finally, sulfuric acid is added in each well to stop the enzymatic reaction, which will cause the oxidized TMB to turn yellow. The absorbance of this yellow product is measured at 450 nm and the signal is correlated to the concentration of MMP-9. A standard curve is constructed by plotting absorbance values against concentrations of standards. The concentration of the biomarker in analysed samples are determined using this standard curve.
Materials provided & Storage conditions
|
|
DESCRIPTION |
Storage |
Ref. |
Qty |
|
① |
MMP-9 Microplate: 96 well microplate (12 strips of 8 wells) coated with a mouse monoclonal antibody against MMP-9. |
|
CTP-BP-02 |
96-w microtiter plate |
|
② |
Human MMP-9 standard: 180 ng of recombinant, lyophilized human MMP-9 / vial. |
STD-BP-02 |
1 vial |
|
|
③ |
Mouse monoclonal antibody (Detection Antibody): 125 µl (80-fold concentrated solution)/ vial |
DTA-BP-02 |
1 vial |
|
|
④ |
Streptavidin-HRP: 80 µL of HRP (100-fold concentrated solution) /vial. |
FLS-HRP03-02 |
1 vial |
|
|
⑤ |
10x Wash Buffer: 50mL |
FLS-WSB-03 |
1 vial |
|
|
⑥ |
TMB substrate: 12 mL of tetramethylbenzidine/vial |
FLS-TMB01 |
1 vial |
|
|
⑦ |
Stop Solution: 6mL of 2N sulfuric acid/vial |
FLS-SPS01 |
1 vial |
|
|
⑧ |
QC high Human recombinant MMP9 |
QCH-BP-02 |
1 vial |
|
|
⑨ |
QC low Human recombinant MMP9 |
QCL-BP-02 |
1 vial |
|
|
/ |
Plate Sealers 12 strips |
|
N/A |
5 units |
Other material required
- Deionized or distilled water
- Test tubes for dilution of standards and samples
- Appropriate glassware for dilutions
- Precision pipettes and appropriate tips
- Multichannel pipette
- Reservoirs
- Paper towels and aluminium foil
- Microplate washer (Optional)
- Orbital microplate shaker.
- Vortex
- Microplate reader to measure absorbance at 450 nm
Sample collection, Storage and Preparation
Sample preparation:
Use a serum separator tube and allow samples to clot for 30 minutes at room temperature before centrifuging for 15 minutes at 1000 g. Isolate serum and use it immediately or aliquot and store serum samples at ≤ -80 °C. Avoid repeated freeze-thaw cycles. Serum samples require a 180-fold dilution in 1X Wash Buffer before measurement. Sample stability has not been evaluated.
Reagent preparation
- Bring all reagents to room temperature.
- Prepare the quantity of reagents needed for the test.
- Do not use components after the expiration date marked on their label.
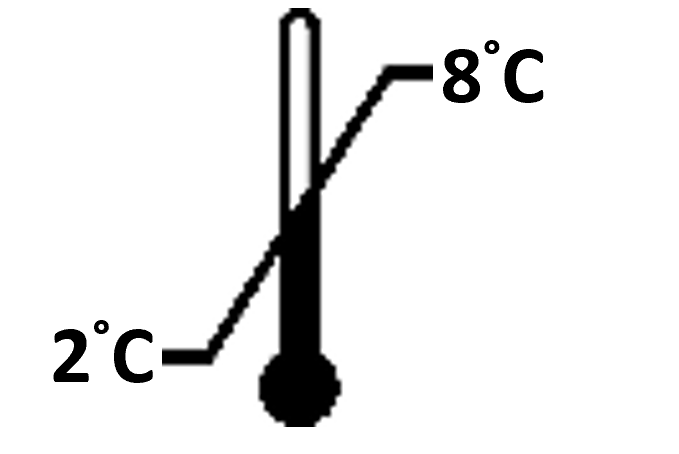
10X Wash Buffer ⑤: is supplied as a 10X concentrated solution. Before the test, warm to room temperature and mix gently until crystals have completely dissolved. To prepare 1X Wash Buffer dilute the concentrated solution 10X ⑤ in deionized water. (e.g. dilute 50 mL of 10X Wash Buffer in deionized water to obtain a final volume of 500 mL). The diluted wash buffer is stable for 1 year when stored at 2-8 °C. Opened 10X Wash buffer is stable until its expiration date (see label on the bottle) when stored at 2-8°C.
Standard ②: the MMP-9 Standard should be reconstituted in 1 mL of distilled water at least 15 min prior the assay with occasional shaking. Harsh mixing and pipetting may be harmful for the integrity of the solution and should be avoided. The resulting concentration of MMP-9 in the stock solution is 180 ng/mL. Do not store the reconstituted vial. Sealed lyophilized vial is stable until its expiration date (see label on the vial) when stored at 2-8°C.
Proceed with the preparation of the 7 calibrators by serial dilutions with freshly prepared 1X Wash Buffer. The 180 ng/mL calibrator serves as the highest calibrator (stock solution). 1X Wash Buffer serves as the zero calibrator (0 ng/mL). The table below explains how to construct the calibration curve in duplicate.
|
Volume of standard |
Volume of 1X Wash Buffer |
Concentration |
|
50 µL of stock solution |
250 µL |
30 ng/mL |
|
150 µL of 30 ng/mL |
150 µL |
15 ng/mL |
|
150 µL of 15 ng/mL |
150 µL |
7.50 ng/mL |
|
150 µL of 7.50 ng/mL |
150 µL |
3.75 ng/mL |
|
150 µL of 3.75 ng/mL |
150 µL |
1.875 ng/mL |
|
150 µL of 1.875 ng/mL |
150 µL |
0.9375 ng/mL |
|
150 µL of 0.9375 ng/mL |
150 µL |
0.47 ng/mL |
Note: It is recommended to use a precision pipette and a careful technique to perform the dilutions in order to get precise results.
Mix each tube before the next transfer. Prepared calibrators are ready to use, do not further dilute them. Do not store diluted solutions.
Coated Microplate ①, TMB substrate ⑥ and Stop solution ⑦ are provided ready to use. Opened TMB substrate and Stop solution are stable until their expiration date (see label on bottles) when stored at 2-8°C. Be careful to return the unused strips of the plate to the aluminium zip-sealed bag with desiccant and seal. Remaining strips are stable 1 month when stored at 2-8°C and protected from moisture. Sealed components are stable until their expiration date (see label on the considered reagent) when stored at 2-8°C.
Detection antibody ③ is supplied as a 80X concentrated solution. To prepare the working solution of detection antibody, dilute the concentrated solution 80X ③ in 1X Wash Buffer. For example, dilute 75 µL of 80X concentrated Detection Antibody in 1X Wash Buffer to a final volume of 6 mL. Opened concentrated antibody is stable until its expiration date (see label on the bottle) when stored at 2-8°C. Do not store the diluted detection antibody.
Streptavidin-HRP ④ is supplied as a 100X concentrated solution. To prepare the working solution of Streptavidin-HRP, dilute the concentrated solution 100X ④ in 1X Wash Buffer. For example, dilute 60 µL of 100X concentrated Streptavidin-HRP in 1X Wash Buffer to a final volume of 6 mL. Opened concentrated Streptavidin-HRP is stable until its expiration date (see label on the bottle) when stored at 2-8°C. Do not store the diluted streptavidin-HRP.
Quality controls High ⑧ and Low ⑨ are provided as lyophilized. Reconstitute each vial with 1 mL of 1X Wash Buffer. Let it dissolve at least 15 min with occasional shaking. Harsh mixing and pipetting may be harmful for the integrity of the solution and should be avoided. The resulting concentration of MMP-9 in the QC High and QC Low solutions are respectively within [7.2-10.8] ng/mL and [1.9-2.8] ng/mL. Do not store the reconstituted vial. Otherwise, sealed lyophilized vials are stable until their expiration date (see label on the vial) when stored at 2-8°C.
Each testing laboratory should establish a quality control program to monitor the performance of the human MMP-9 ELISA test.
Assay procedure
Required time: 4 hours.
Bring all reagents and samples to room temperature before use. It is recommended that all standards and controls be assayed in duplicate.
- Prepare all reagents, working standards and samples as indicated in the previous section.
- Remove excess microplate strips from the plate frame, return them to the foil pouch containing the desiccant pack, and reseal.
- Wash the wells once with wash buffer (250 µL per well).
- Pipet 50 µL of standards, quality controls and diluted samples into the appropriate wells. Cover the plate with provided sealer.
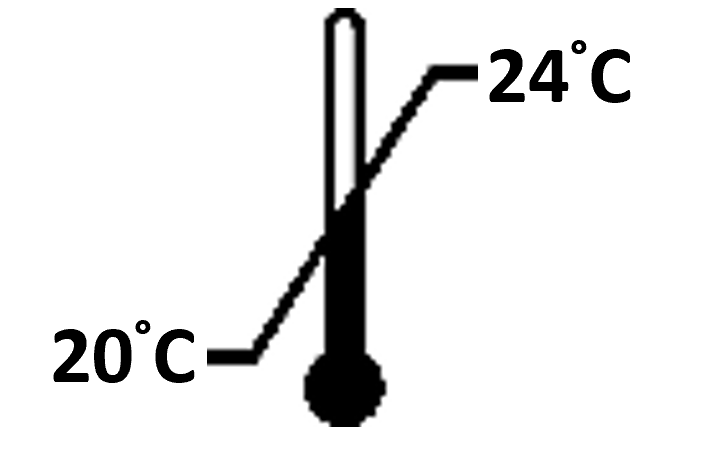
- Incubate the plate for 2 hours at room temperature (20-25°C), at 600 rpm on an orbital microplate shaker.
- Wash the wells 3 times with wash buffer (250 µL per well). After the last wash, invert the plate and blot it against clean paper towels.
- Add 50 µL of diluted detection antibody into each well. Cover the plate with provided sealer.
- Incubate the plate for 1 hour at room temperature (20-25°C), at 600 rpm on an orbital microplate shaker.
- Wash three times as in step 6.
- Add 50 µL of diluted streptavidin-HRP into each well. Cover the plate with provided sealer.
- Incubate the plate for 30 minutes at room temperature (20-25°C), at 600 rpm on an orbital microplate shaker.
- Wash three times as in step 6.
- Add 100 µL of TMB substrate to each well. Protect from light.
- Incubate the plate in the dark for 10 min, at room temperature (20-25°C).
- Stop the reaction by adding 50 µL of stop solution. The colour in wells changes from blue to yellow. Gently tap the plate to ensure correct mixing.
- Read the optical density of each well using a microplate reader set to 450 nm. The optical density should be read within 5 min after step 15.
If wavelength correction is available, set to 540 nm or 570 nm. If wavelength correction is not available, subtract readings at 540 nm or 570 nm from the readings at 450 nm. This subtraction will correct for optical imperfections in the plate. Readings made directly at 450 nm without correction may be higher and less accurate.
Note: For manual washing, aspirate carefully the contents of the wells, avoiding to touch the bottom of the well with the tip, and pipet 250 µL wash buffer into each well. Repeat two times more. After final washing step, invert and tap the plate against paper towel. Make sure that wash buffer has been completely removed.
Note: If samples generate values higher than the highest standard, further dilute the samples and repeat the assay.
Note: High concentrations of MMP-9 are found in saliva. Use a face mask and gloves to protect kit reagents from contamination.
Calculation and Interpretation of Results
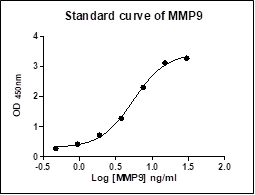
Calculate the average of duplicate readings, if applied, for each standard, control, and sample. The standard curve is constructed by plotting the absorbance (Y) of standards against the known concentration (X) of standards on a logarithmic scale, using a four-parameter algorithm. For sample analysis, raw absorbance values are interpolated and the concentrations are calculated. Results are reported as concentration of MMP-9 (ng/mL) in samples. The measured concentration in samples calculated from the standard curve must be multiplied by their respective dilution factor (DF).
Technical Advice
- When mixing or reconstituting protein solutions, pipet gently to avoid foaming.
- Improper or insufficient washing at any stage of the procedure will result in either false positive or false negative results. Empty wells completely before dispensing fresh wash solution, fill with Wash Buffer as indicated for each wash cycle and do not allow wells to sit uncovered or dry for extended time periods.
- Avoid any contamination among samples and reagents. For this purpose, change tips at each step. It is also recommended to use separate reservoirs for each reagent.
- Bacterial or fungal contamination of either screen samples or reagents or cross-contamination between reagents may cause erroneous results.
- Avoid stacking plates.
- To obtain accurate results, ensure that plate sealers are airtight during incubation steps.
- The TMB Substrate is light sensitive. Keep away from bright light. Once added to the plate, TMB solution should change from colourless to gradations of blue.
- Stop solution should be added to the plate after addition of TMB substrate. The colour developed in the wells will turn from blue to yellow.
- Dispose of consumable materials and unused contents in appropriate containers.
Liability
- This kit is only intended for the in vitro determination of human MMP-9 in human serum samples.
- The kit is only intended to be used by qualified personnel carrying out research. If the recipient of this kit passes on in any way to a third party, this instruction must be enclosed, and in this case, Firalis do not guarantee the product performances.
- Firalis shall not be responsible for any damages or losses due to using the kit in any way other than as expressly stated in these instructions.
- Firalis is not responsible for any patent infringements that might result from the use or derivation of this product.
Warning and Method Limitations
- This assay is designed to quantify MMP-9 in human serum samples, within the assay quantification range.
- The kit should not be used beyond the expiration date indicated on the kit label.
- Do not mix or substitute reagents with those from other lots or sources.
- If samples generate values higher than the highest standard or ULOQ value, further dilute the samples with 1X Wash Buffer and repeat the assay.
- Use only clean glassware.
- Use deionized water stored in clean containers.
- Any variation in washing buffer, operator, pipetting technique, washing technique, incubation time or temperature, can cause variations in assay precision and accuracy.
- Variations in sample collection, processing, and storage may cause sample value differences.
Performance characteristics
This standard curve is provided for demonstration only. A standard curve should be generated for each set of samples assayed.
Tracability of calibrator value
No internationally approved reference material for MMP-9 is currently available.
Assay performance
The analytical performance evaluation is based on the Firalis’ internal protocol which follows COFRAC guidelines on validation of biological methods, “GUIDE TECHNIQUE D'ACCREDITATION DE VERIFICATION (PORTEE A) / VALIDATION (PORTEE B) DES METHODES EN BIOLOGIE MEDICALE” The objective of the validation is to demonstrate that an analytical method is suitable for its intended purpose.
1.Detection range
Low limit of quantification (LLOQ) is the lowest measured value for which the %CV is equal or below 20 % CV.
For this assay, 11 serial dilutions of the calibrator with the lowest concentration of analyte: measurement of 6 replicates for each dilution were performed.
Upper limit of quantification (ULOQ) is the upper value for which the %CV is equal or below 10% CV. For this assay, 11 serial dilutions of the calibrator with the highest concentration of analyte: measurement of 6 replicates for each dilution were performed.
|
LLOQ (ng/mL) |
LLOQ x DF (ng/mL) |
ULOQ (ng/mL) |
ULOQ x DF (ng/mL) |
|
1 |
180 |
22 |
3960 |
If results obtained are out of the ULOQ level, the assay should be repeated with more diluted samples. Dilution factor needs to be taken into account when calculating the MMP-9 concentration.
2.Limit of detection (LOD)
The limit of detection (LOD) or sensitivity is defined as the lowest protein concentration that is significantly different from a blank sample. The LOD was determined by adding 3 SD to the mean value of 30 replicates of 1X Wash Buffer analysed in the same run. The corresponding concentration was then calculated.
The minimum detectable concentration of MMP9 is 0.1 ng/ml.

Thank you for choosing Firalis
- No drugs have been investigated for assay interference.
- Since conditions may vary from assay to assay, a standard curve must be established for each run. Do not re-use the wells of the plate in any case.
- Most solutions in this ELISA kit are not ready-for-use but have to be prepared by the user.
Precaution
Biological samples may be used by qualified persons, using appropriate protections (Lab coat, double gloves and safety glasses) and appropriate equipment. Samples have to be handled as potentially infectious to prevent all risks. In case of accidental blood exposure, contact a physician.”
User Safety
 The Stop Solution
The Stop Solution
![]() This solution is an acid solution. Wear protective gloves, clothing, eye, and face protection. Wash hands thoroughly after handling. Upon skin contact wash with plenty of water.
This solution is an acid solution. Wear protective gloves, clothing, eye, and face protection. Wash hands thoroughly after handling. Upon skin contact wash with plenty of water.
For more information, please refer to the Material Safety Data Sheet (MSDS).
Troubleshooting
On the occasion of an assay failure, check the expiration dates of the individual reagents and ensure that all the reagents have been stored as indicated in the product label. If assay performance is questionable or a problem occurs when running the assay, you may be able to isolate the problem by referring to the following table:
For further technical information, contact aftersales@firalis.com
|
Problem |
Possible Source |
|
Inadequate Colour Development |
Pipetting error Dilution factor error Error in the incubation times TMB substrate is contaminated |
|
Poor Curve Fit |
Pipetting error Dilution factor error |
|
Poor Precision |
Pipetting error Dilution factor error Washing step missing or incomplete |
![]()
3.Precision
a.Intra-assay precision (Repeatability) (precision within an assay).2 samples with low (2.4 ng/mL) and high (9 ng/mL) level of MMP-9 were tested 30 times on a single plate.
|
Samples |
n |
Mean ng/mL |
%CV |
|
LOW |
30 |
2.6 |
3.50 |
|
HIGH |
30 |
8.7 |
5.58 |
The CV of repeatability is within ± 15% which is an acceptable range.
b.Inter-assay precision: 2 samples with low (2.4 ng/mL) and high (9 ng/mL) level of MMP-9 were obtained under normal analysis conditions from separated runs and different batches, on different days and/or operators.
|
Samples |
n |
Mean ng/mL |
%CV |
|
LOW |
10 |
2.7 |
2.5 |
|
HIGH |
10 |
9.5 |
1.1 |
The CV of repeatability is within ± 20% which is an acceptable range.
4.Linearity
The linearity was assayed by testing a serum sample with known concentration of MMP-9 and its serial dilution. The linearity was demonstrated by the recovery (percentage of calculated concentration to the expected).
|
DF |
140 |
160 |
180 |
200 |
220 |
240 |
260 |
|
Expected |
7.14 |
6.25 |
5.56 |
5 |
4.55 |
4.17 |
3.85 |
|
Measured |
8.3 |
6.9 |
6 |
5.4 |
4.9 |
4.6 |
4.2 |
|
Recovery (%) |
115.9 |
109.8 |
107.3 |
108.4 |
107.8 |
109.5 |
110.4 |
Samples can be diluted between 140 and 260-fold with an acceptable recovery range of 80-120%
Symbol Legend
|
|
Catalogue number |
|
|
Batch code |
|
|
Temperature limitations |
|
|
Consult instructions for use |
|
|
Biological risk |
|
|
Warning |
|
|
Warning, Corrosive product |
For any further information, MSDS or other languages Instructions enquiries, please contact us:
aftersales@firalis.com
References
- Heymans S, Luttun A, Nuyens D, et al. Inhibition of plasminogen activators or matrix metalloproteinases prevents cardiac rupture but impairs therapeutic angiogenesis and causes cardiac failure. Nat Med. 1999;5(10):1135-1142. doi:10.1038/13459
- Romanic AM, Burns-Kurtis CL, Gout B, Berrebi-Bertrand I, Ohlstein EH. Matrix metalloproteinase expression in cardiac myocytes following myocardial infarction in the rabbit. Life Sci. 2001;68(7):799-814. http://www.ncbi.nlm.nih.gov/pubmed/11205871. Accessed November 23, 2018.
- Rohde LE, Ducharme A, Arroyo LH, et al. Matrix metalloproteinase inhibition attenuates early left ventricular enlargement after experimental myocardial infarction in mice. Circulation. 1999;99(23):3063-3070. http://www.ncbi.nlm.nih.gov/pubmed/10368126. Accessed August 29, 2018.
- LINDSEY M, GOSHORN D, SQUIRES C, et al. Age-dependent changes in myocardial matrix metalloproteinase/tissue inhibitor of metalloproteinase profiles and fibroblast function. Cardiovasc Res. 2005;66(2):410-419. doi:10.1016/j.cardiores.2004.11.029
- Chiao YA, Dai Q, Zhang J, et al. Multi-analyte profiling reveals matrix metalloproteinase-9 and monocyte chemotactic protein-1 as plasma biomarkers of cardiac aging. Circ Cardiovasc Genet. 2011;4(4):455-462. doi:10.1161/CIRCGENETICS.111.959981
- Ducharme A, Frantz S, Aikawa M, et al. Targeted deletion of matrix metalloproteinase-9 attenuates left ventricular enlargement and collagen accumulation after experimental myocardial infarction. J Clin Invest. 2000;106(1):55-62. doi:10.1172/JCI8768